Dual role of drug makers: taking a stand for humanity, conducting business
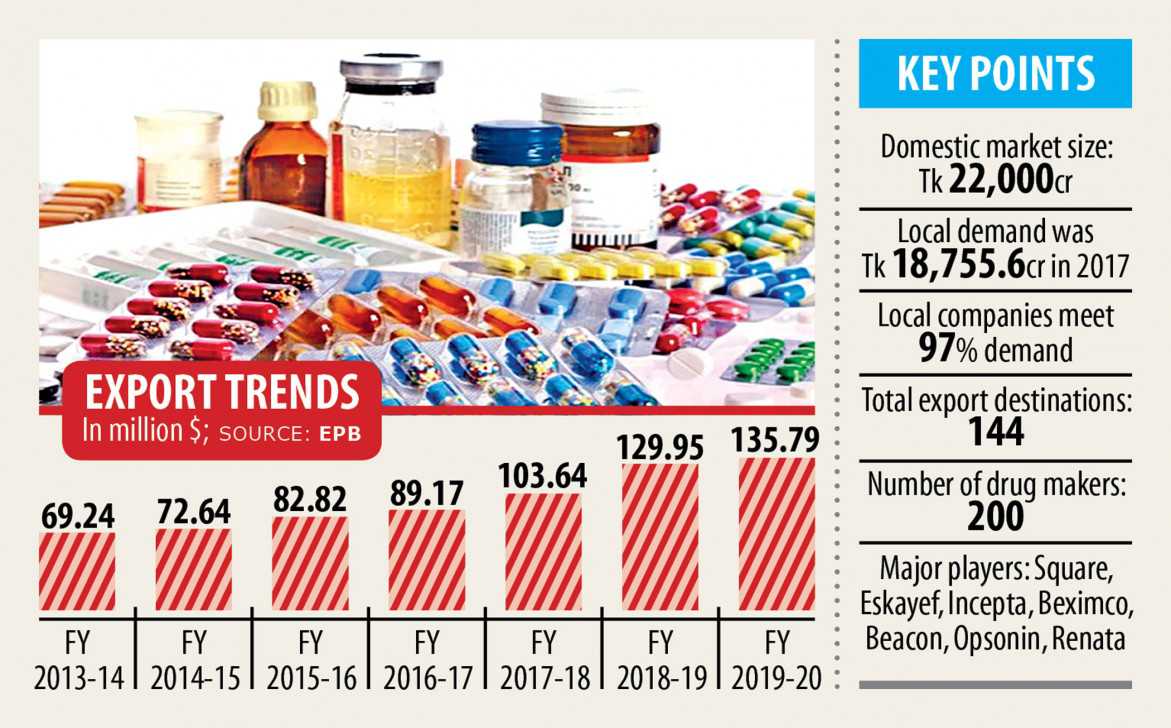
Image collected
Bangladesh's pharmaceuticals sector is managing never to only play its supportive role amidst the ongoing Covid-19 pandemic but also maintain business growth, all as a result of its compliance with international standards in terms of product quality, cite industry insiders with pride.
"The pharmaceuticals sector passed a terrible time through the lockdown to continue daily work," said Syed Golam Rahman, managing director of Lab Aid Pharmaceuticals.
"Fortunately the sector were able to manufacture drugs for supportive treatment though there is absolutely no specific medicine for Covid-19 patients on the globe," he said.
Rahman was addressing a webinar on "Pharmaceutical sector under Covid-19 spotlight: how it can seize work at home opportunities and perform social responsibilities" organised by The Daily Star on Sunday evening.
The sector spent some time working hard since the emergence of the pandemic, particularly its marketing section, to attain necessary medicine to remote regions of the country, he said.
According to him, the pharmaceuticals sector catered to 97 per cent of the domestic demand and soon would meet up with the remaining 3 %.
"The industry flourished and there's a quality gap between domestic and multinational companies," noted Rahman.
The caliber of products is ensured through selecting globally renowned manufacturers when importing active pharmaceutical ingredients (API), he also said.
The pharmaceuticals industry dominates the domestic market and today it is time to make an impression on the global market, said Ahmed Kamrul Alam, director for marketing at Square Pharmaceuticals.
"The pharmaceuticals industry is highly regulated, so there is absolutely no scope to compromise with quality of the merchandise," he said.
"Now we have to utilise our economic power by buying research and development to create our own version of medicines consistent with generic versions," he said.
He believes the sector would further develop later on and for this the government's cooperation was required. Regarding product quality, he said company size had not been a factor, rather it had been performance and product quality.
Several small companies are performing well, competing with bigger peers by ensuring product quality, said Alam.
The standard of drugs can not solely secure effective treatment, as there is also the necessity for ensuring proper dosages, he further said.
The pharmaceutical companies of Bangladesh proved that there surely is no difference in quality between your original and generic versions, he said.
The sector works under intensive surveillance of the Directorate General of Drug Administration (DGDA) and World Health Organisation, so there is no reason for the initial and generic versions to vary, Alam said.
The quality of drugs of Bangladesh is equivalent to that of the united states, UK and Europe, said EH Arefin Ahmed, general manager for marketing at Incepta Pharmaceuticals.
The importers audit the quality of products and manufacturing facilities each year to maintain the standard, he said.
Bangladesh is creating around 1,000 pharmacists every year and the companies are receiving manpower at low-cost compared to developed countries, which will take the country quite a distance in the future, he also said.
He emphasised on maintaining reasonable prices of products while maintaining quality to ensure that those stayed affordable to the masses.
Regarding the product quality, he said Bangladesh's companies were exporting drugs to 144 countries through their tested quality and by securing approvals from global regulatory bodies.
During the pandemic, the firms focused on exporting Remdesivir, which has been proven to be effective in treating serious Covid-19 cases, because they had the opportunity to offer reasonable prices alongside product quality, he said.
Quality control during distribution is important, especially in case there is some temperature sensitive medications, otherwise their effectiveness will certainly deteriorate, said Lucky Singha, head of business at Sanofi.
In cases like this, manufacturers aren't responsible, rather the distribution system is, she noted.
She emphasised the necessity for pharmacies to keep good practices, including ensuring preservation of medicines under required temperatures, to guard quality and effectiveness.
Public health specialist Tune Tehrin conducted the webinar.
Source: https://www.thedailystar.net