US FDA clears Vaporox’s VHT-200 wound care medical device
Collected Image
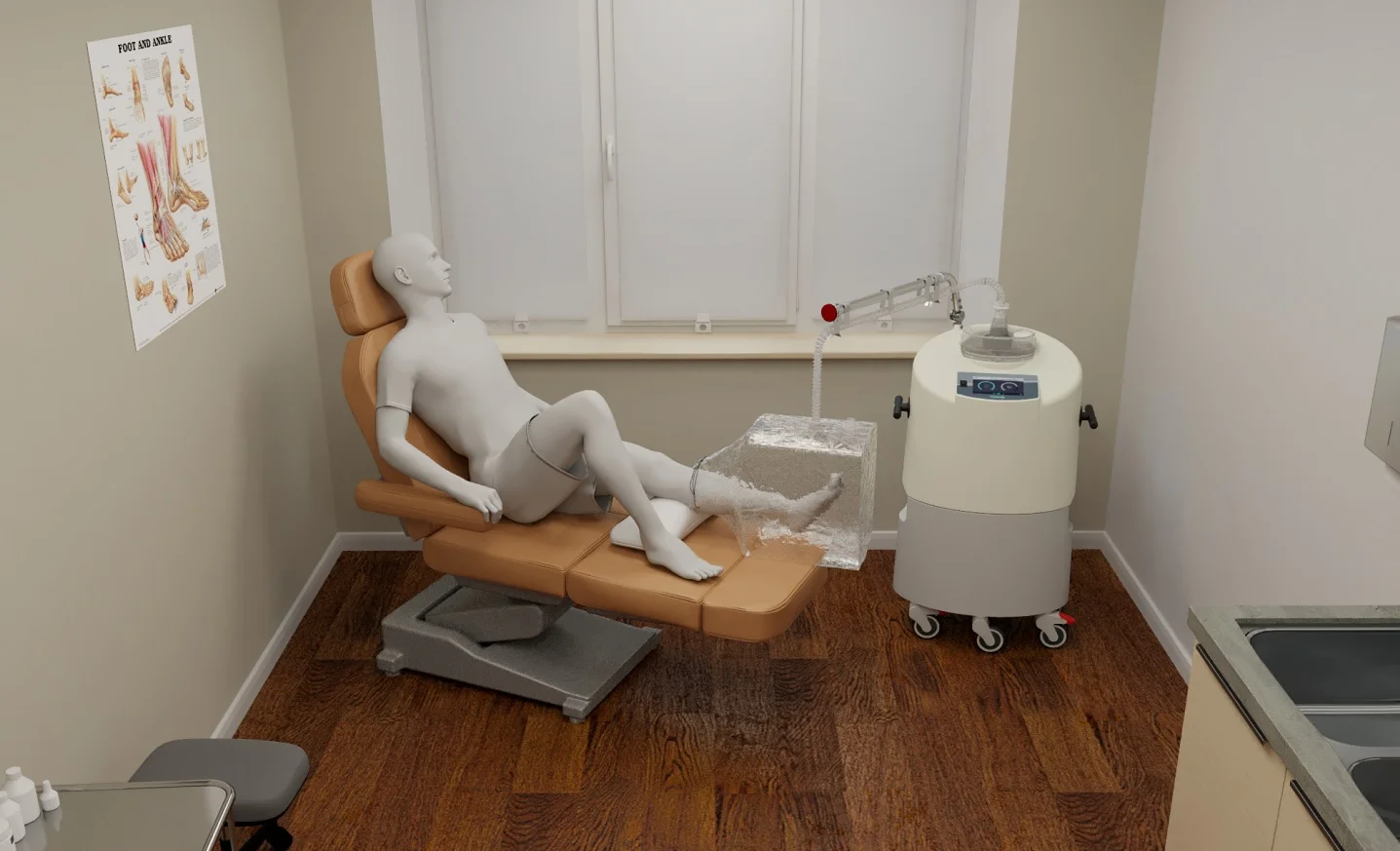
The US Food and Drug Administration (FDA) has granted clearance for Vaporox’s next-generation VHT-200 medical device system to heal chronic wounds. The patented, FDA 510(k)-cleared breakthrough technology Vaporous Hyperoxia Therapy (VHT) is a low-frequency, non-contact, non-thermal ultrasound treatment.
It has been clinically validated as a safe and effective adjunctive treatment to heal nine types of skin wounds. VHT uniquely combines ultrasonic mist and concentrated oxygen, two elements that have been shown to expedite healing. The system uses these elements to promote revascularisation and tissue growth.
Vaporox stated that the next-generation VHT-200 medical device system brings advanced wound care technology into the private practice setting.
It provides healing rates for chronic wounds of more than 80% and allows doctors to provide advanced wound care in their practice.
Vaporox Board of Directors chairman Louis Woodhill said: “It has been profoundly satisfying to have played a role in bringing this lifesaving and life-changing technology to market. “The VHT-200 represents a major step forward toward a world where all wounds heal.”
According to the company, approximately 30 million Americans have diabetes, with more than two million suffering from diabetic foot ulcers (DFUs). Furthermore, nearly 550,000 new cases of DFU are diagnosed in the country every year.
Vaporox’s VHT was found to heal 70%-85% of DFUs, which are classed as chronic wounds that resist standard treatment, in several clinical studies and thousands of commercial treatments.
The company intends to immediately start placing the VHT-200 devices in private practices with podiatrists and MDs in the US.
It has been clinically validated as a safe and effective adjunctive treatment to heal nine types of skin wounds. VHT uniquely combines ultrasonic mist and concentrated oxygen, two elements that have been shown to expedite healing. The system uses these elements to promote revascularisation and tissue growth.
Read More : Why Nanomaterials Are Used In Medical Devices
According to three separate clinical trials and many commercial treatments, the addition of VHT to standard wound care doubles or triples healing and delivers results in 20 weeks or less.Vaporox stated that the next-generation VHT-200 medical device system brings advanced wound care technology into the private practice setting.
It provides healing rates for chronic wounds of more than 80% and allows doctors to provide advanced wound care in their practice.
Vaporox Board of Directors chairman Louis Woodhill said: “It has been profoundly satisfying to have played a role in bringing this lifesaving and life-changing technology to market. “The VHT-200 represents a major step forward toward a world where all wounds heal.”
According to the company, approximately 30 million Americans have diabetes, with more than two million suffering from diabetic foot ulcers (DFUs). Furthermore, nearly 550,000 new cases of DFU are diagnosed in the country every year.
Vaporox’s VHT was found to heal 70%-85% of DFUs, which are classed as chronic wounds that resist standard treatment, in several clinical studies and thousands of commercial treatments.
The company intends to immediately start placing the VHT-200 devices in private practices with podiatrists and MDs in the US.
Source: https://www.medicaldevice-network.com
Previous Story
- Healthcare financing needs attention
- Belmont Medical Technologies Donates Lifesaving Medical Equipment to...
- CSSi LifeSciences branches out medical device CRO
- The world’s largest medical device companies
- Medical supplies company CCS moves into home-based diabetes...
- FDA adds AEDs and other medical devices to...
- Nanotechnology in Medicine: Regulatory trends
- 4 key challenges in sustaining compliance in the...